Home > Science > Concepts of Physics 101 > Atoms and Electrons
Atoms and Electrons
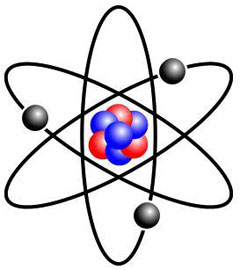
Electricity involves forces at the very basis of the structure of atoms that make up our physical world. Matter and elements surrounding us are all made of atoms. An atom has a nucleus at its centre that contains neutrons and protons. Negatively charged particles known as electrons revolve around the central nucleus. Protons, equal in number to electrons, have a positive charge and neutrons, as their name indicates, are neutral electrically.
The nucleus of an atom forms much of its mass. The diameter of the atom is 100 000 time greater than its nucleus. Thus, one can deduce that a large part of an atom involves empty space. Electrons spin around the nucleus. If one were to view the nucleus as an orange, the electron the furthest away from the nucleus would be located at a distance of seven kilometres.
The word atom signifies indivisible, meaning it cannot be divided. It origin is attributed to a Greek philosopher born in Milet around 460 B.C. According to this philosopher, matter is formed of tiny, indivisible particles invisible to the eye. One of his disciples, Democritus, further developed this theory before it was completely forgotten.
The theory of Democritus would be reinstated nearly 2000 years later by British chemist and physicist John Dalton. In 1801, his study on the composition of gases, and air in particular, led to the formulation of the first modern atomic theory based on observation and experimentation. He formulated the hypothesis that matter was made up of particles that he deemed indivisible.
Another British physicist, Joseph John Thomson, pushed the atomic theory even further with the discovery of the electron in 1897. Thanks to his experiments, he was able to demonstrate that the atom was composed of positive and negative charges.
In 1911, a student of Thomson, New Zealander Ernest Rutherford, who also worked for several years at McGill University in Montréal, showed that the atom effectively featured a very dense nucleus composed of protons. Rutherford also proved that electrons spin around the nucleus at a relatively great distance, effectively leaving a great deal of empty space. He compared his model to the solar system where electrons, like the planets, rotate around the sun, which he compared to the nucleus. In 1932, British physicist James Chadwick discovered the neutron, a particle of the nucleus of the atom with no electrical charge.